Products
Fexofenadine Hydrochloride
Fexofenadine Hydrochloride is a second-generation antihistamine, used to treat allergy symptoms, such as hay fever and urticaria, marketed by Sanofi S.A. as Allegra with 25 DMF and 16 MF approved before 2021 Q3.
Prochem can provide pilot sample as white powder with above 99.5% purity.
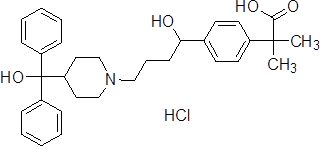
Product Name: Fexofenadine Hydrochloride
CAS Number: 153439-40-8
Indication: Hay fever / urticaria
Specification: JP 17 / USP 42 (for related compound B)
Certificate: None
Non-infringement Description:
- US6743941B2 is original substance patent of Fexofenadine Hydrochloride, and will expire on 2022.06.11.
- JP2005502603A is family patent of US6743941B2 in Japan, and already cancelled due to non-payment of annual fee.
- No related patent submitted in Taiwan.
- In this situation, no infringement concerns arise after 2022.06.11.

