Products
Ertapenem Sodium
Ertapenem Sodium is a carbapenem antibiotics medication for the abdomen, the lungs, the upper part of the female reproductive system, and diabetic foot infections, marketed by Merck & Co. as Invanz with 8 DMF approved before 2021 Q3.
Prochem implemented process validation with batch size above 5 KG in 2021, and can provide finished product as white to light yellow powder with above 96.9% purity.
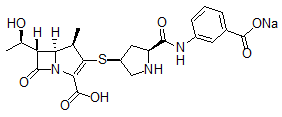
Product Name: Ertapenem Sodium
CAS Number: 153773-82-1
Indication: Antibiotic agent
Specification: USP Draft (Residual solvents can meet the specification of ICH Guideline)
Certificate: None
Non-infringement Description:
- US5478820A is original substance patent of Ertapenem Sodium, and already expired on 2015.11.21.
- JP2730600B2 is family patent of US5478820A in Japan, and already expired on 2013.02.02.
- TW408124B is family patent of US5478820A in Taiwan, and already expired on 2013.02.09.
- In this situation, no infringement concerns arise.

