Products
Lansoprazole
Lansoprazole is a proton pump inhibitor, used to treat peptic ulcer disease and gastroesophageal reflux disease, marketed by Takeda Pharmaceutical Co. Ltd. as Prevacid with 62 DMF and 18 MF approved before 2021 Q3.
Prochem implemented process validation with batch size above 50 KG in 2020, and can provide finished product as white powder with above 99.90% purity.
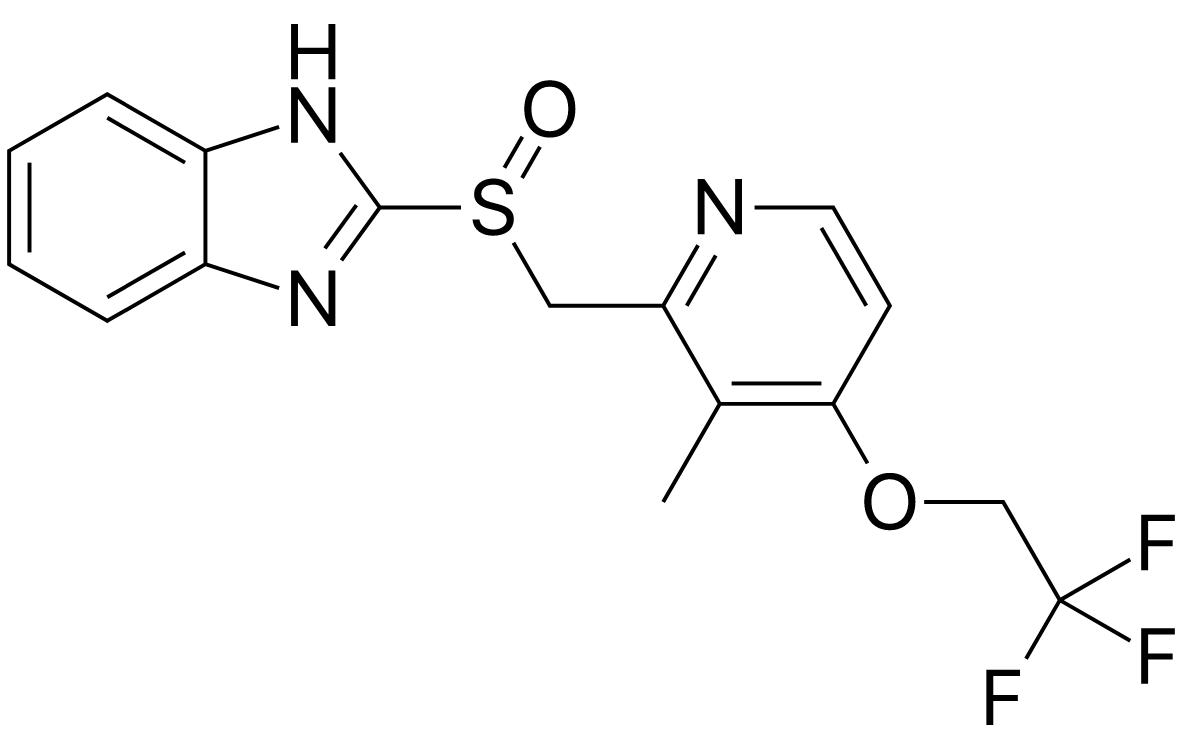
Product Name: Lansoprazole
CAS Number: 103577-45-3
Indication: Peptic ulcer disease / gastroesophageal reflux disease
Specification: JP 17
Certificate: PIC/S GMP certificate of bay factory / drug exportation certificate
Non-infringement Description:
- US4628098A and US4689333A are original substance patents of Lansoprazole, and already expired on 2009.05.10 and 2005.07.29.
- JPS6150978A is family patent of US4628098A and US4689333A in Japan, and already expired on 2008.12.08.
- No related patent submitted in Taiwan.
- In this situation, no infringement concerns arise.

